Publications of M-STAR citing Center for Chemical Innovation support
This work is supported by funding from the U.S. National Science Foundation under Grant Number CHE-2318105 (M-STAR CCI).
2025
19. Chenkun Zhou, Young-Hwan Kim, Benjamin Atterberry, Vikash Khokhar, Arashdeep S. Thind, Francisco Lagunas, Ruiming Lin, Di Wang, Wooje Cho, Zirui Zhou, Maia E. Czaikowski, Alexander S. Filatov, John S. Anderson, Robert F. Klie, Richard D. Schaller, De-en Jiang, Aaron J. Rossini, and Dmitri V. Talapin. MXenoids: Generalization of MXene-Inspired Covalent Surface Modifications Across Two-Dimensional Materials. Journal of the American Chemical Society 2025, Article ASAP.
Abstract
The ability to perform versatile covalent surface modifications in two-dimensional (2D) inorganic materials marks a significant advance in the functionalization of this broad family of materials. One particularly successful example of 2D materials with chemically modifiable surfaces are 2D transition metal carbides and nitrides (MXenes). MXenes’ strong in-plane metal–carbon bonds and labile surface metal–halide bonds create altogether unprecedented opportunities for versatile postsynthetic modifications and assembling complex materials, including various organic–inorganic hybrids. We demonstrate the general applicability of this surface modification strategy to non-MXene halide-terminated 2D materials, termed MXenoids. These surface modifications enable compositional and electronic structure engineering, introduce chiral hybrid organic–inorganic structures, and photoluminescence ranging from near-IR to blue. This study highlights the avenue of surface chemistry-driven materials design, enhancing the functional capabilities of 2D materials.

19. Anupma Thakur, Nithin Chandran B.S., Brian C. Wyatta Annabelle Bedford, Mostafa Dadashi Firouzjaei, Krutarth Kamath, Muhammad Sharif Uddin, and Babak Anasori. Six key variables in 2D MXenes for hydrogen evolution electrocatalysis. Materials Today 2025, Article ASAP.
Abstract
MXenes, with their Mn+1XnTx chemical formula, have diverse chemical compositions and structures among two dimensional (2D) materials. MXenes have emerged as potential electrocatalysts for hydrogen evolution reaction (HER) due to their abundant active sites on the 2D basal planes and tunable electronic properties. MXenes with two or more transition metals are growing subfamilies of MXenes, including random solid solutions, ordered double-metal, and multi-metal (four or more metal) MXenes. Computational studies indicate that ordered double transition metal (DTM) MXenes (o-MXenes) offer superior catalytic HER performance compared to mono-transition metal MXenes. In this article, we first present six key factors that play critical roles in the tunability and control of the HER performance of MXenes, including composition (M, X, and Tx), the thickness of 2D flake (number of atomic layers), lateral flake dimensions, surface adatoms, and intercalating ions, atomic defects (e.g., vacancies), and heteroatom doping. We then systematically evaluate the effect of transition metals (M) on MXenes HER activity by analyzing 14 single-metal and ordered DTM MXenes, including four M2CTx as Mo2CTx, V2CTx, Nb2CTx, Ti2CTx, four M3C2Tx as Ti3C2Tx, Mo2TiC2Tx, Cr2TiC2Tx, W2TiC2Tx and six M4C3Tx as V4C3Tx, Nb4C3Tx, Ta4C3Tx, Mo2Ti2C3Tx, Mo2Nb2C3Tx and Mo2V2C3Tx. By focusing on the role of M in MXenes, with Ti, V, Cr, Nb, Mo,Ta, and W as their inner and outer planes, we provide a comprehensive understanding of their effects on HER activity. Among all screened MXenes, W2TiC2Tx MXene shows the lowest HER overpotential of ∼149 mV at 10 mA/cm2 under acidic conditions, which could be attributed to the active W-basal plane. This work provides insights into the interplay of six compositional and structural factors in MXene-based HER and highlights the critical influence of transition metal selection in guiding the development of high-performance MXene electrocatalysts.

18. Tetiana Parker, Yuan Zhang, Kateryna Shevchuk, Teng Zhang, Vikash Khokhar, Young-Hwan Kim, Givi Kadagishvili, David Bugallo, Manushree Tanwar, Ben Davis, Jongyoun Kim, Zahra Fakhraai, Yong-Jie Hu, De-en Jiang, Dmitri V. Talapin, and Yury Gogotsi. In Situ Raman and Fourier Transform Infrared Spectroscopy Studies of MXene−Electrolyte Interfaces. ACS Nano 2025, 19, 24, 22228–22239.
Abstract
A comprehensive understanding of electrochemical interfaces is essential for the optimal performance of electrocatalysts, supercapacitors, and batteries. However, understanding the electrochemical behavior of MXenes during electrochemical processes by any single technique does not provide a whole picture. We achieved real-time monitoring in the complete near-mid-infrared chemical range by utilizing Raman spectroscopy (near-infrared (NIR) excitation) and Fourier transform infrared (FTIR) spectroscopy in the mid-infrared (MIR) range. The change of intramolecular O−H vibrations of MXene-confined water was monitored in real time using FTIR, while surface terminations were monitored by using Raman spectroscopy. The dynamic interplay between charge storage and the change in MXene surface chemistry was studied by employing representative electrolytes (0.5 M H2SO4, 1 M LiCl, and 6 M KOH) and comparing hydrophilic Ti3C2Tx with mixed-terminations (T = O/OH/F) with hydrophobic chlorine-terminated Ti3C2Cl2 MXene electrodes. Ab initio molecular dynamics (MD) simulations and density functional theory (DFT) calculations were used to shed light on ion insertion with a dynamic change of ion solvation and reveal the structure of the MXene-confined water.
Link: https://pubs.acs.org/doi/full/10.1021/acsnano.5c03810h

17. Anupma Thakur, Yuan Zhang, Babak Anasori, and Yury Gogotsi. Electrochemistry of MXenes and their Sustainable Energy Applications. MRS Energy and Sustainability 2025, 12, 1. (Review article)
Abstract
MXenes, a growing class of two-dimensional (2D) materials, have garnered significant attention for their unique electrochemical properties and diverse applications in sustainable energy technologies. Their metallic electrical conductivity, large surface area, tunable surface chemistry, and 2D nanoconfinement make them promising candidates for energy storage devices, including supercapacitors and batteries, and emerging electrochemical applications, such as electrocatalysis and green fuel production. In addition, their inherent mechanical strength and flexibility position them as key materials for flexible and wearable energy harvesting and storage devices. This perspective highlights the structural and chemical tunability of MXenes, emphasizing how these factors control their electrochemical properties and their potential to address global energy challenges through sustainable and innovative applications.
Link: https://link.springer.com/article/10.1557/s43581-025-00130-9

16. Francisco Lagunas, Chenkun Zhou, Di Wang, Anupma Thakur, Babak Anasori, Dmitri V. Talapin, Zachary D. Hood, Robert F. Klie. In Situ Formation of Ripplocations in Hybrid Organic–Inorganic MXenes. Advanced Materials 2025, 37, 13, 2411669.
Abstract
Inorganic–organic hybrid MXenes (h-MXenes) are a family of 2D transition metal carbides and nitrides functionalized with alkylimido and alkylamido surface groups. Using cryogenic and room temperature scanning transmission electron microscopy (STEM) and electron energy-loss spectroscopy (EELS), it is shown that ripplocations, a form of a fundamental defect in 2D and layered structures, are abundant in this family of materials. Furthermore, detailed studies of electron probe sample interactions, focusing on structural deformations caused by the electron beam are presented. The findings indicate that at cryogenic temperatures (≈100 K) and below a specific dose threshold, the structure of h-MXenes remains largely intact. However, exceeding this threshold leads to electron beam-induced deformation through ripplocations. Interestingly, the deformation behavior, required dose, and resultant structure are highly dependent on temperature. At 100 K, it is demonstrated that the electron beam can induce ripplocations in situ with a high degree of precision.
Link: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adma.202411669

15. James FitzPatrick, Sumit Bera, Alex Inman, Alessandra Cabrera, Teng Zhang, Tetiana Parker, Bita Soltan Mohammadlou, Iryna Roslyk, Stefano Ippolito, Kateryna Shevchuk, Sujit A. Kadam, Nihar R. Pradhan, and Yury Gogotsi. Record Efficiency of β-Phase PVDF-MXene Composites in Thin-Film Dielectric Capacitors. Advanced Materials 2025, 37, 12, 2419088.
Abstract
Polyvinylidene fluoride (PVDF) is a semicrystalline polymer used in thin-film dielectric capacitors because of its inherently high dielectric constant and low loss tangent. Its dielectric constant can be increased by the formation and alignment of its β-phase crystalline structure, which can be facilitated by 2D nanofillers. 2D carbides and nitrides, MXenes, are promising candidates due to their notable dielectric permittivity and ability to increase interfacial polarization. Still, their mixing is challenging due to weak interfacial interactions and poor dispersibility of MXenes in PVDF. This work explores a novel method for delaminating Ti3C2Tx MXene directly into organic solvents while maintaining flake size and quality, as well as the use of a non-solvent-induced phase separation method for producing both dense and porous PVDF-MXene composite films. A deeper understanding of dielectric behavior in these composites is reached by examining MXenes with both mixed and pure chlorine terminations in PVDF matrices. Thin-film capacitors fabricated from these composites display ultrahigh discharge energy density, exceeding 45 J cm−3 with 95% efficiency. The PVDF-MXene composites are also processed using a green and sustainable solvent, propylene carbonate.
Link: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adma.202419088

14. Janek Rieger, Atreyie Ghosh, Joseph Spellberg, Calvin Raab, Aishani Mohan, Prakriti Joshi, and Sarah King. Imaging and Simulation of Surface Plasmon Polaritons on Layered 2D MXenes. Science Advances 2025, 11, 12, eads3689. (Research materials provided by M-STAR.)
Abstract
Two-dimensional (2D) transition metal carbides and nitrides, commonly known as MXenes, are a class of 2D materials with high free carrier densities, making them highly attractive candidates for plasmonic 2D materials. In this study, we use multiphoton photoemission electron microscopy (nP-PEEM) to directly image the plasmonic near fields of multilayers of the prototypical MXene, Ti3C2Tx, with mixed surface terminations (Tx = F, O, and OH). Photon-energy dependent nP-PEEM reveals a dispersive surface plasmon polariton between 1.4 and 1.9 electron volts on MXene flakes thicker than 30 nanometers and waveguide modes above 1.9 electron volts. Combining experiments with finite-difference time-domain simulations, we reveal the emergence of a visible surface plasmon polariton in MXenes, opening avenues for exploration of polaritonic phenomena in MXenes in the visible portion of the electromagnetic spectrum.
Link: https://www.science.org/doi/full/10.1126/sciadv.ads3689
Preprint link: https://chemrxiv.org/engage/chemrxiv/article-details/66ba644a5101a2ffa81dc23b

13. Andreas Furchner, Tetiana Parker, Vincent Mauchamp, Simon Hurand, Julian Plaickner, Jörg Rappich, Aline Alencar Emerenciano, Karsten Hinrichs, Yury Gogotsi, and Tristan Petit. Ti3C2Tx MXene Thin Films and Intercalated Species Characterized by IR-to-UV Broadband Ellipsometry. Journal of Physical Chemistry C 2025 129, 1, 500–507.
Abstract
MXenes are two-dimensional (2D) materials with versatile applications in optoelectronics, batteries, and catalysis. To unlock their full potential, it is crucial to characterize MXene interfaces and intercalated species in more detail than is currently possible with conventional optical spectroscopies. Here, we combine ultra-broadband ellipsometry and transmission spectroscopy from the mid-infrared (IR) to the deep-ultraviolet (UV) to probe quantitatively the composition, structure, transport, and optical properties of spray-coated Ti3C2Tx MXene thin films with varying material properties. We find film thickness heterogeneity and surface roughness in the low-nanometer range as well as depth-dependent conductivity properties, which we quantify with a graded Drude model. The optically determined sheet resistance is confirmed by four-point probe measurements. Furthermore, we employ density-functional-theory calculations to assign the observed absorption bands in the MXene dielectric function to various interband transitions from mixed MXene surface terminations. The prominent 1.48 eV (833 nm) spectral feature is found to be related to oxygen termination. Additional plasmonic effects are also suggested. Finally, we leverage the chemical sensitivity of state-of-the-art IR ellipsometry to separate the fingerprints of intercalated species within the MXene from the dominant Drude contributions, presenting for the first time a set of infrared optical constants of intercalated water. This work lays the foundation for optical metrology for interface engineering of MXene and other 2D materials.
Link: https://pubs.acs.org/doi/full/10.1021/acs.jpcc.4c06906

2024
12. Yubin Huang, Jean Spiece, Tetiana Parker, Asaph Lee, Yury Gogotsi, and Pascal Gehring. Violation of the Wiedemann–Franz Law and Ultralow Thermal Conductivity of Ti3C2Tx MXene. ACS Nano 2024 18, 47, 32491–32497.
Abstract

11. Ervin Rems, Yong-Jie Hu, Yury Gogotsi, and Robert Dominko. Pivotal Role of Surface Terminations in MXene Thermodynamic Stability. Chemistry of Materials 2024 36, 20, 10295-10306.
Abstract
MXenes, i.e., two-dimensional transition metal carbides and nitrides, have been reported as promising materials for various applications, including energy storage, biomedicine, and electronics. The family of MXenes has proliferated, and the chemical space of synthesized MXenes has expanded to 13 transition metals and a dozen elements in surface terminations. The diverse chemistry of MXenes enables systematical tuning of MXene properties to meet the needs of target applications. However, synthesizing new MXene compositions largely relies on a trial-and-error approach. To overcome it, computational predictions of MXene compositions that are thermodynamically stable are crucial to rationalize experimental efforts. Here, we report a comprehensive computational screening for thermodynamically stable MXenes across 29 transition metals and 11 surface terminations. Density functional theory calculations are employed to compute the energy above the convex energy hull as a descriptor of thermodynamic stability. The results are analyzed to explore factors crucial for determining the thermodynamic stability of MXenes, by which the chemistry of surface terminations is found to play a crucial role. The insights on the chemistry of 998 MXene compositions predicted to be (meta)stable are given to systematically guide further research on MXene synthesis and application.
Link: https://pubs.acs.org/doi/10.1021/acs.chemmater.4c02274

10. Chaofan Chen, Glenn Quek, Hongjun Liu, Lars Bannenberg, Ruipeng Li, Jaehoon Choi, Dingding Ren, Ricardo Javier Vázquez, Bart Boshuizen, Bjørn-Ove Fimland, Simon Fleischmann, Marnix Wagemaker, De-en Jiang, Guillermo Carlos Bazan, and Xuehang Wang. High-Rate Polymeric Redox in MXene-Based Superlattice-Like Heterostructure for Ammonium Ion Storage. Advanced Energy Materials 2024 14, 42, 2402715.
Abstract
Link: https://onlinelibrary.wiley.com/doi/full/10.1002/aenm.202402715

9. Alex Inman, Tetiana Parker, Yuan Zhang, Mohit Saraf, Yury Gogotsi. Obtaining a Practical Wearable Supercapacitor Power Supply. Advanced Energy Materials 2024 14, 43, 2402367.
Abstract
Link: https://onlinelibrary.wiley.com/doi/full/10.1002/aenm.202402367

8. Mengni Jiang, Di Wang, Young-Hwan Kim, Chunying Duan, Dmitri V. Talapin, and Chenkun Zhou. Evolution of Surface Chemistry in Two-Dimensional MXenes: From Mixed to Tunable Uniform Terminations. Angewandte Chemie International Edition 2024 63, 37, e202409480.
Abstract
Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202409480

7. Tetiana Parker, Danzhen Zhang, David Bugallo, Kateryna Shevchuk, Marley Downes, Geetha Valurouthu, Alex Inman, Benjamin Chacon, Teng Zhang, Christopher E. Shuck, Yong-Jie Hu, and Yury Gogotsi. Fourier-Transform Infrared Spectral Library of MXenes. Chemistry of Materials 2024 36, 17, 8437-8446.
Abstract
Fourier-transform infrared (FTIR) spectroscopy characterization is a powerful and easy-to-use technique frequently employed for the characterization and fingerprinting of materials. Although MXenes are a large and fastest growing family of inorganic 2D materials, the lack of systematic FTIR spectroscopy studies hinders its application to MXenes and often leads to misinterpretation of the results. In this study, we report experimental and calculated FTIR spectra of 12 most typical carbide and carbonitride MXenes with different compositions (5 transition metals) and all four basic structures, including Ti2CTx, Nb2CTx, Mo2CTx, V2CTx, Ti3C2Tx, Ti3CNTx, Mo2TiC2Tx, Mo2Ti2C3Tx, Nb4C3Tx, V4C3Tx, Ta4C3Tx, and Mo4VC4Tx. The measurements were performed on delaminated MXene flakes incorporated in KBr pellets in the 4000–400 cm–1 range. We provide detailed instructions for sample preparation, data collection, and interpretation of FTIR spectra of MXenes. Background correction and spectra smoothing are applied to obtain clear FTIR peaks corresponding to bond vibrations in MXenes. Density functional theory calculations were used for the precise assignment of all characteristic FTIR peaks and an in-depth analysis of the vibration modes. This work aims to provide the 2D material community with the FTIR spectroscopy technique as a reliable method for identifying and analyzing MXenes.
Link: https://pubs.acs.org/doi/full/10.1021/acs.chemmater.4c01536

6. Geetha Valurouthu, Mikhail Shekhirev, Mark Anayee, Ruocun (John) Wang, Kyle Matthews, Tetiana Parker, Robert W. Lord, Danzhen Zhang, Alex Inman, Marley Downes, Chi Won Ahn, Vibha Kalra, Il-Kwon Oh, Yury Gogotsi. Screening Conductive MXenes for Lithium Polysulfide Adsorption. Advanced Functional Materials 2024 34, 45, 2404430.
Abstract
MXenes are promising passive components that enable lithium-sulfur batteries (LSBs) by effectively trapping lithium polysulfides (LiPSs) and facilitating surface-mediated redox reactions. Despite numerous studies highlighting the potential of MXenes in LSBs, there are no systematic studies of MXenes’ composition influence on polysulfide adsorption, which is foundational to their applications in LSB. Here, a comprehensive investigation of LiPS adsorption on seven MXenes with varying chemistries (Ti2CTx, Ti3C2Tx, Ti3CNTx, Mo2TiC2Tx, V2CTx, Nb2CTx, and Nb4C3Tx), utilizing optical and analytical spectroscopic methods is performed. This work reports on the influence of polysulfide concentration, interaction time, and MXenes’ chemistry (transition metal layer, carbide and carbonitride inner layer, surface terminations and structure) on the amount of adsorbed LiPSs and the adsorption mechanism. These findings reveal the formation of insoluble thiosulfate and polythionate complex species on the surfaces of all tested MXenes. Furthermore, the selective adsorption of lithium and sulfur, and the extent of conversion of the adsorbed species on MXenes varied based on their chemistry. For instance, Ti2CTx exhibits a strong tendency to adsorb lithium ions, while Mo2TiC2Tx is effective in trapping sulfur by forming long-chain polythionates. The latter demonstrates a significant conversion of intermediate polysulfides into low-order species. This study offers valuable guidance for the informed selection of MXenes in various functional components benefiting the future development of high-performance LSBs.
Link: https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202404430

5. Marley Downes, Christopher E. Shuck, Bernard McBride, Jeffrey Busa, and Yury Gogotsi. Comprehensive synthesis of Ti3C2Tx from MAX phase to MXene. Nature Protocols 2024 19, 1807-1834.
Abstract
MXenes are a large family of two-dimensional materials that have attracted attention across many fields due to their desirable optoelectronic, biological, mechanical and chemical properties. There currently exist many synthesis procedures that lead to differences in flake size, defects and surface chemistry, which in turn affect their properties. Herein, we describe the steps to synthesize Ti3C2Tx—the most important and widely used MXene, from a Ti3AlC2 MAX phase precursor. The procedure contains three main sections: synthesis of Ti3AlC2 MAX, wet chemical etching of the MAX in hydrofluoric acid/HCl solution to yield multilayer Ti3C2Tx and its delamination into single-layer flakes. Three delamination options are described; these use LiCl, tertiary amines (tetramethyl ammonium hydroxide/ tetrabutyl ammonium hydroxide) and dimethylsulfoxide respectively. These procedures can be adapted for the synthesis of MXenes beyond Ti3C2Tx. The MAX phase synthesis takes about 1 week, with the etching and delamination each requiring 2 d. This protocol requires users to have experience working with hydrofluoric acid, and it is recommended that users have experience with wet chemistry and centrifugation; characterization techniques such as X-ray diffraction and particle size analysis are also essential for the success of the protocol. While alternative synthesis methods, such as minimally intensive layer delamination, are desirable for certain MXenes (such as Ti2CTx) or specific applications, this protocol aims to standardize the more commonly used hydrofluoric acid/HCl etching method, which produces Ti3C2Tx with minimal concentration of defects and the highest conductivity and serves as a guideline for those working with MXenes for the first time.

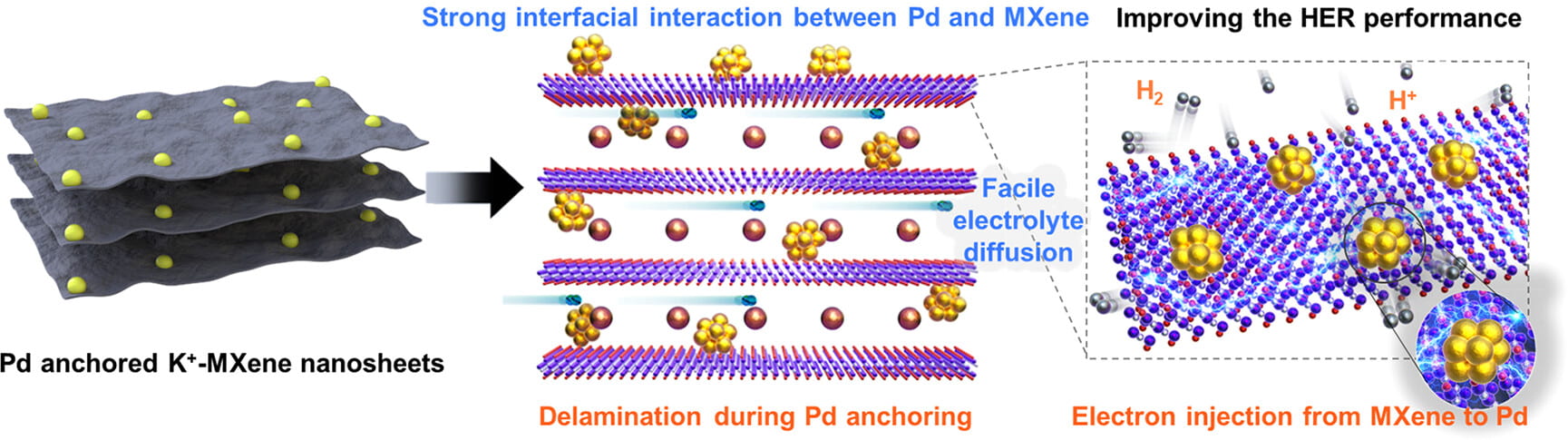
4. Yiyang Sun, Jihyeong Lee, Nam Hee Kwon, Joohyun Lim, Xiaoyan Jin, Yury Gogotsi, and Seong-Ju Hwang. Enhancing Hydrogen Evolution Reaction Activity of Palladium Catalyst by Immobilization on MXene Nanosheets. ACS Nano 2024 18, 8, 6243–6255.
Abstract
Efficient catalysts with minimal content of catalytically active noble metals are essential for the transition to the clean hydrogen economy. Catalyst supports that can immobilize and stabilize catalytic nanoparticles and facilitate the supply of electrons and reactants to the catalysts are needed. Being hydrophilic and more conductive compared with carbons, MXenes have shown promise as catalyst supports. However, the controlled assembly of their 2D sheets creates a challenge. This study established a lattice engineering approach to regulate the assembly of exfoliated Ti3C2Tx MXene nanosheets with guest cations of various sizes. The enlargement of guest cations led to a decreased interlayer interaction of MXene lamellae and increased surface accessibility, allowing intercalation of Pd nanoparticles. Stabilization of Pd nanoparticles between interlayer-expanded MXene nanosheets improved their electrocatalytic activity. The Pd-immobilized K+-intercalated MXene nanosheets (PdKMX) demonstrated exceptional electrocatalytic performance for the hydrogen evolution reaction with the lowest overpotential of 72 mV (@10 mA cm–2) and the highest turnover frequency of 1.122 s–1 (@ an overpotential of 100 mV), which were superior to those of the state-of-the-art Pd nanoparticle-based electrocatalysts. Weakening of the interlayer interaction during self-assembly with K+ ions led to fewer layers in lamellae and expansion of the MXene in the c direction during Pd anchoring, providing numerous surface-active sites and promoting mass transport. In situ spectroscopic analysis suggests that the effective interfacial electron injection from the Pd nanoparticles strongly immobilized on interlayer-expanded PdKMX may be responsible for the improved electrocatalytic performance.
3. Teng Zhang, Kateryna Shevchuk, Ruocun John Wang, Hyunho Kim, Jamal Hourani, and Yury Gogotsi. Delamination of Chlorine-Terminated MXene Produced Using Molten Salt Etching. Chemistry of Materials 2024 36, 4, 1998–2006.
Abstract
MXenes produced by Lewis acid molten salt (LAMS) etching of MAX phases have attracted the community’s attention due to their controllable surface chemistry. However, their delamination is challenging due to the hydrophobicity of the produced multilayer MXene and strong interactions between the halogen-terminated MXene sheets. The current delamination method involves dangerous chemicals such as n-butyllithium or sodium hydride, making scale-up difficult and limiting the practical application of this class of MXenes. In this work, we present a simple and efficient method for the delamination of MXenes from the LAMS synthesis while maintaining their surface chemistry. LiCl salt and anhydrous polar organic solvents are used for delamination. Films produced from the delaminated MXene are flexible and have an electrical conductivity of 8000 S/cm, which is maintained after a week of exposure to 95% humidity. This successful delamination, preservation of inherent surface properties, and stability under high-humidity conditions dramatically expand the range of MXene chemistries available for research and potential applications.
Link: https://pubs.acs.org/doi/full/10.1021/acs.chemmater.3c02872

2023
2. Yury Gogotsi. The Future of MXenes. Chemistry of Materials 2023 35, 21, 8767-8770. (Editorial)
Excerpt
We are entering the era of new materials: materials that can be assembled from nanoscale building blocks, unlike all previous material generations from the Stone Age to the Silicon Age. Two-dimensional (2D) materials provide nanometer and subnanometer-thin “bricks” for such assembly. If needed, organic molecules and polymers can serve as mortar, but van der Waals (vdW) or electrostatic forces can also provide a strong bonding between the 2D layers. To make this vision real and start assembling materials, structures, and devices from nanoparticles, we need many building blocks with a large variety of physical and chemical properties. … The advancement of MXenes toward industrial use would require the development of scalable, low-cost, safe, and environmentally friendly synthesis processes, as well as extensive and thorough studies of their toxicity and fate in the environment. All new materials follow this path. Based on the unique and tunable properties of MXenes as well as their enormous compositional diversity and tunability of properties, we are confident that the field of MXenes has a bright and exciting future.
Link: https://pubs.acs.org/doi/full/10.1021/acs.chemmater.3c02491
1. Babak Anasori and Yury Gogotsi. The global expansion of MXenes. Graphene and 2D Materials 2023 8, 39-41. (Editorial, open-access)
Excerpt
Since their discovery in 2011, the family of 2D transition metal carbides and nitrides, MXenes, has made remarkable progress. While their initial development was relatively slow compared to their current growth and some other 2D materials, MXenes have gained momentum over the past seven years. We can expect accelerating progress as the critical mass of scientists and engineers focusing on MXenes is being accumulated. Still, since MXenes are developing into the largest known family of inorganic low-dimensional materials with an enormously broad range of applications in almost every engineering field, as well as healthcare and analytical chemistry, there is plenty of room for discovery for any number of researchers entering the field of these fascinating materials. Moreover, considering the transformational role that MXenes are expected to play in the fields from communication to optoelectronics, healthcare, energy and environment, countries that seize the opportunity earlier may gain a major technological advantage.
Link: https://link.springer.com/article/10.1007/s41127-023-00067-1
0. Chenkun Zhou, Di Wang, Francisco Lagunas, Benjamin Atterberry, Ming Lei, Huicheng Hu, Zirui Zhou, Alexander S. Filatov, De-en Jiang, Aaron J. Rossini, Robert F. Klie, and Dmitri V. Talapin. Hybrid organic–inorganic two-dimensional metal carbide MXenes with amido- and imido-terminated surfaces. Nature Chemistry 2023 15, 1722-1729. (Published before designation of award number by M-STAR)
Excerpt
Two-dimensional (2D) transition-metal carbides and nitrides (MXenes) combine the electronic and mechanical properties of 2D inorganic crystals with chemically modifiable surfaces, which provides an ideal platform for both fundamental and applied studies of interfaces. Good progress has been achieved in the functionalization of MXenes with small inorganic ligands, but relatively little work has been reported on the covalent bonding of various organic groups to MXene surfaces. Here we synthesize a family of hybrid MXenes (h-MXenes) that incorporate amido- and imido-bonding between organic and inorganic parts by reacting halogen-terminated MXenes with deprotonated organic amines. The resulting hybrid structures unite tailorability of organic molecules with electronic connectivity and other properties of inorganic 2D materials. Describing the structure of h-MXene necessitates the integration of concepts from coordination chemistry, self-assembled monolayers and surface science. The optical properties of h-MXenes reveal coherent coupling between the organic and inorganic constituents. h-MXenes also exhibit superior stability against hydrolysis.
Link: https://link.springer.com/article/10.1007/s41127-023-00067-1

