What are MXenes?
The name MXene (pronounced M-X-ene, max-ene or M-zene according to taste) arises from their chemical formula, Mn+1XnTy, where M is an early transition metal (most commonly Ti, Zr, Hf, V, or Nb) and X is carbon or nitrogen. MXenes originally were named by way of analogy to the intensively-studied graphene; the terminating group T has since been recognized as an crucial part of the chemical formula of most MXene compositions, and commonly refers to chalcogens (O, S, Se, Te) and halides (F, Cl, Br, I). The structure of an MXene sheet is quite simple: n+1 M layers are intercalated with the n X layers and covered with the T groups. The simplicity of this picture belies the complexity and range of MXene properties, and it is M-STAR’s goal to set this enormous chemical space on a sound footing.
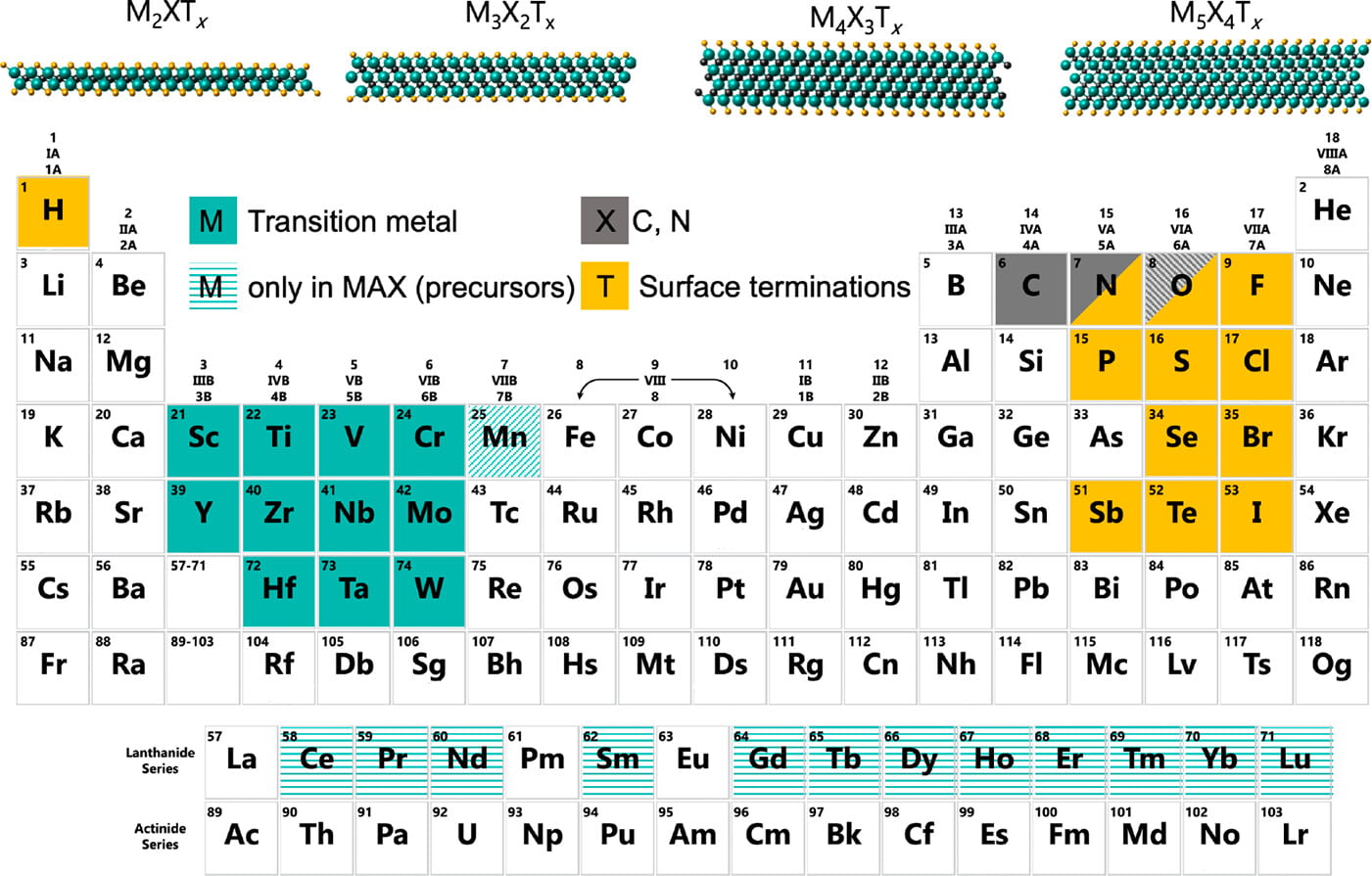
The components of MXenes and some of their structures. Link

A pure metal carbide, Ti2AlC, withstands forceful blows from a hammer that would shatter most ceramics. (Image is 20 cm across.) Link
MXenes are unusual among the many families of two-dimensional materials: they were originally discovered and studied by materials scientists and engineers, rather than chemists and physicists. Early transition metal carbides and nitrides have long been among the hardest and most refractory compounds known. They are essential in anti-wear coatings, machine tools, and structural materials, but have one deficiency: like most superhard substances, they are brittle. Engineering research on carbides, after many decades, culminated in the work of Barsoum and others on ternary carbides possessing the M and X elements and a third element, this time from the early p block of the periodic table (commonly Al, Si, Ga, and Ge). The component became known by the abbreviation A and the ternary carbides as the MAX phases. These carbides and nitrides retained the stiffness, electrical conductivity, and thermal stability of the simpler compounds while being highly workable and damage tolerant.
Key to MAX phase properties was their layered structure: for example, Ti3AlC2 consists of Ti3C2 (Ti-C-Ti-C-Ti) layers alternating with single Al layers. The resemblance of these layers to the two-dimensional compounds such as graphene and molybdenum disulfide, which were currently undergoing a frenzy of research in the latter half of the 2000s, did not go unnoticed by those studying MAX phases. They then undertook their own research program attempting to tease apart the metal carbide layers into thin free-standing sheets. Barsoum and Gogotsi (now a founding member of M-STAR) finally succeeded in 2011 with the preparation of exfoliated Ti3C2 nanosheets from Ti3AlC2. The necessary separation was provided by using aqueous hydrofluoric acid to etch the Al layers away from the carbide sheets. The method proved to be extendable to many different compounds, and since then MXene research has grown explosively.

Transmission electron microscopy of exfoliated Ti3C2(OH)x, the first discovered MXene. (a-b) Single flakes seen supported on thin carbon films. (c-e) Restacked MXenes seen along their basal planes. (f) Schematic of the structure as seen in the high-resolution TEM. Link
